Goal and Focus
A formal working group within CPIC, PharmGKB, PharmVar, and the PGRN was formed in 2018 to focus on the dissemination of resources available for pharmacogenetic implementation such as CPIC guidelines, PharmGKB, PGRN and PharmVar resources, and implementation strategies of pharmacogenetics. The goal of the CPIC Dissemination Working Group is to support the dissemination of these resources to clinicians by identifying opportunities to engage clinicians and payors and raise awareness of these resources.
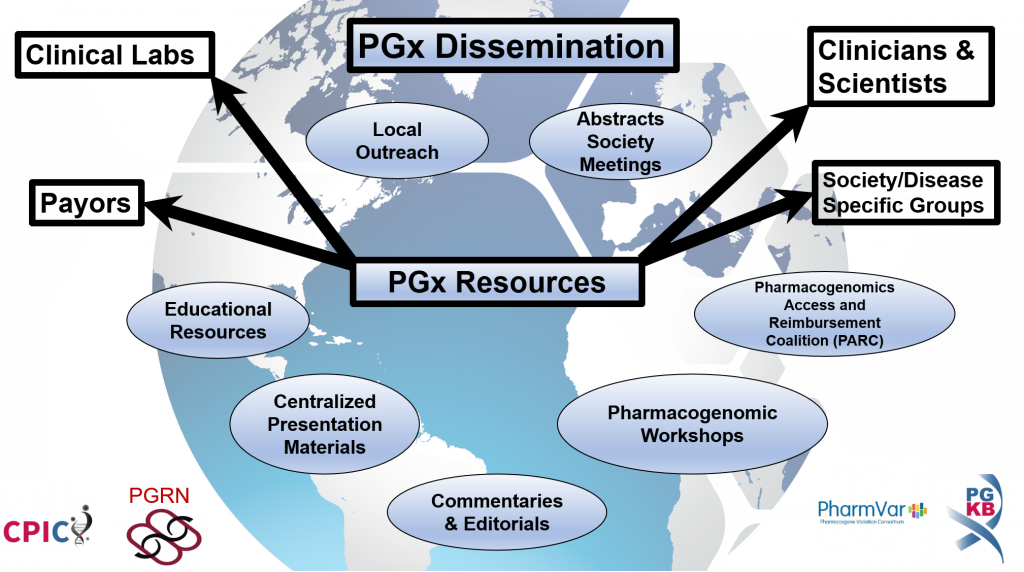
Organization and Membership
The PGx Dissemination Working Group is a subgroup within the CPIC. As such, the working group reports to CPIC leadership and provides regular updates during CPIC meetings. The working group co-leaders are Andrew Monte, M.D., Ph.D., University of Colorado Denver-Anschutz Medical Center, Daniel Mueller, M.D., Ph.D., University of Toronto and Andria Del Tredici, Ph.D., Millennium Health.
The PGx Dissemination Working Group is open to any CPIC member, and all regular participants must be members of CPIC. As such, all working group members must abide by the CPIC Memorandum of Understanding.
Working group objectives and sub-groups
- Foster relationships with specialty/disease centric organizations (e.g., AHA)
- Coordinate with CPIC/PGRN/PharmGKB/PharmVar members with relationships or leadership roles with these organizations
- Coordinate with specialty guidelines (e.g. AHA, IDSA, ASCO) to include information about the use of pharmacogenetics/CPIC guidelines
- Interact with the Inter-Society Coordinating Committee for Practitioner Education in Genomics (ISCC)
- Develop sessions focused on pharmacogenetic implementation at society conferences
- Submit abstracts at meetings related to the use of CPIC guidelines and PharmGKB knowledgebase tool use for Pgx implementation
- Develop workshops focused on implementation of CPIC guidelines and PharmGKB knowledgebase tools into clinical practice
- Publish journal commentaries on newly released guidelines
- Increase presence of CPIC, PharmGKB, and PGRN links on society websites
- Create increased social media presence
- Interact with the VA Office of research and development, VA hospitals, updates on research
- Create and maintain centralized presentation materials and handouts for use in promoting CPIC
- Increase payor awareness of CPIC guidelines
- Collaborate with the Pharmacogenomics Access and Reimbursement Coalition (PARC)