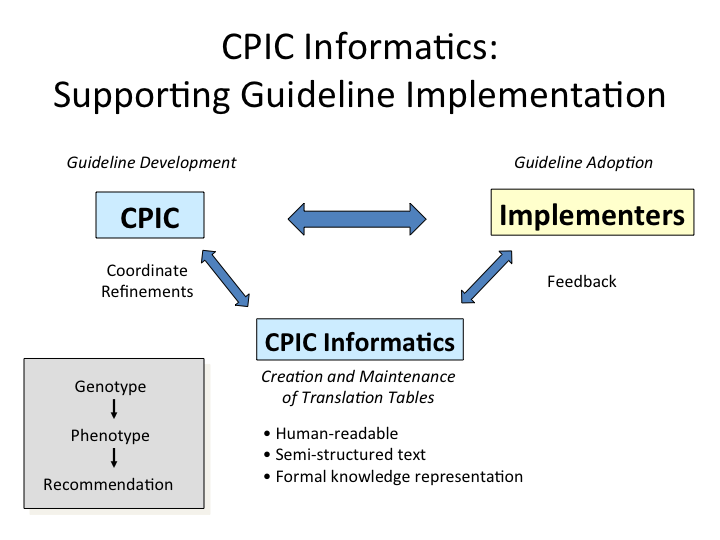
Goal and Focus
A formal working group within CPIC was formed in 2013 to focus on informatics aspects of CPIC guidelines, especially as they relate to the application of the CPIC guidelines in electronic health records (EHRs) with clinical decision support (CDS). The goal of the CPIC Informatics Working Group is to support the adoption of the CPIC guidelines by identifying, and resolving where possible, potential technical barriers to the implementation of the guidelines within a clinical electronic environment.
The primary initial focus for CPIC informatics is to:
- create comprehensive tables and other guidance to translate genotype information to phenotype to clinical recommendation for CPIC guidelines, using human readable and structured text with formal knowledge representation.
- develop recommendations for Clinical Decision Support (CDS) in Electronic Health Records (EHRs) based on the CPIC guidelines.
These resources are being incorporated into the supplement of each new and updated CPIC guideline.
The working group will maintain a relationship with groups (such as eMERGE and members of the PGRN) that are implementing pharmacogenetic testing with CDS. The working group works closely with the authors of CPIC guidelines, especially those implementing PGx rules.
Organization and Membership
The CPIC Informatics Working Group is a subgroup within the CPIC. As such, the working group reports to CPIC leadership and provides regular updates during CPIC meetings. The working group co-leaders are Robert Freimuth, PhD (Mayo Clinic), James Hoffman, PharmD (St. Jude Children’s Research Hospital) and Michelle Whirl-Carrillo, PhD (PharmGKB).
CPIC Informatics members are denoted on the membership page.
The CPIC Informatics Working Group is open to any CPIC member, and all regular participants must be members of CPIC. As such, all working group members must abide by the CPIC Memorandum of Understanding.
Publications and Presentations
The EHR implementation resources that are incorporated into all CPIC guidelines were summarized in JAMIA. This paper also outlines five interlinked principles to define the key features of future knowledge bases for pharmacogenetics.
CPIC implementation resources were also shared with the CDSKB community. This presentation on CDSKB summarizes CPIC’s efforts to support the successful adoption of pharmacogenetics into the EHR (presentation link – The Clinical Pharmacogenetics Implementation Consortium (CPIC): enabling the adoption of pharmacogenetics into the EHR).
The 2014 AMIA Summit on Translational Bioinformatics included the panel “Dissemination of Pharmacogenomic Knowledge: Establishing a Pathway to Support Clinical Implementation.” This presentation describes the origin of CPIC informatics and provides further context on the use of these resources (slides).