Assignment of CPIC Levels for Genes/Drugs
CPIC assigns CPIC levels to gene/drug pairs. The levels (A, B, C, and D) assigned are subject to change; only those gene/drug pairs that have been the subject of guidelines have had sufficient in-depth review of evidence to provide definitive CPIC level assignments.
Note that only CPIC level A and B gene/drug pairs have sufficient evidence for at least one prescribing action to be recommended. CPIC level C and D gene/drug pairs are not considered to have adequate evidence or actionability to have prescribing recommendations.
This video describes CPIC levels and FDA pharmacogenetic information and can be found here.
On this page:
- Assignment of CPIC Levels Diagram
- Level Definitions for CPIC Genes/Drugs
- PharmGKB Level of Evidence (LOE)
- FDA Label Testing
Considerations for Assignment of CPIC Level for Genes/Drugs
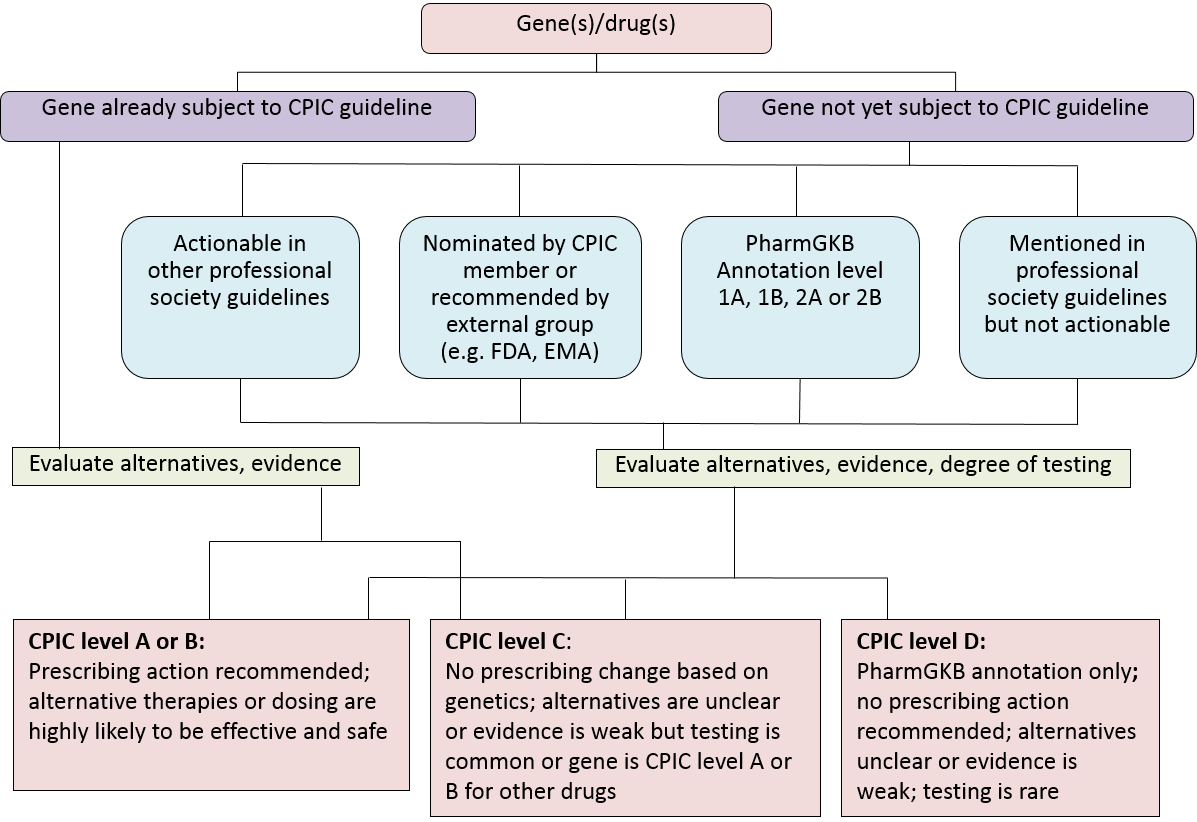
Level Definitions for CPIC Genes/Drugs
| CPIC Level | Clinical Context | Level of evidence | Strength of Recommendation |
|---|---|---|---|
| A | Genetic information should be used to change prescribing of affected drug. | Preponderance of evidence is high or moderate in favor of changing prescribing | At least one moderate or strong action (change in prescribing) recommended. |
| A/B | Preliminary review indicates it is likely that the definitive CPIC level will be either A or B. | Full evidence review needed to assess level of evidence, but prescribing actionability is likely | Full review by expert guideline group to assign strength of recommendation |
| B | Genetic information could be used to change prescribing of the affected drug because alternative therapies/dosing are extremely likely to be as effective and as safe as non-genetically based dosing. | Preponderance of evidence is weak with little conflicting data | At least one optional action (change in prescribing) is recommended. |
| B/C | Preliminary review indicates it is likely that the definitive CPIC level will be either B or C. | Prescribing actionability based on genetics is not clear without further evidence review | Full review by expert guideline group to assess strength of recommendation |
| C | There are published studies at varying levels of evidence, some with mechanistic rationale, but no prescribing actions are recommended because (a) dosing based on genetics makes no convincing difference or (b) alternatives are unclear, possibly less effective, more toxic, or otherwise impractical or (c) few published studies or mostly weak evidence and clinical actions are unclear. Most important for genes that are subject of other CPIC guidelines or genes that are commonly included in clinical or DTC tests. | Evidence levels can vary | No prescribing actions are recommended. |
| C/D | Preliminary review indicates it is likely that the definitive CPIC level will be either C or D. | Evidence levels can vary | No prescribing actions are recommended |
| D | There are few published studies, clinical actions are unclear, little mechanistic basis, mostly weak evidence, or substantial conflicting data. If the genes are not widely tested for clinically, evaluations are not needed. | Evidence levels can vary | No prescribing actions are recommended. |
PharmGKB Level of Evidence (LOE)
PharmGKB curators create Clinical Annotations for gene-drug response pairs that summarize manually curated literature in PharmGKB.Each Clinical Annotation is assigned a Level of Evidence based on multiple criteria including replication, statistical significance and study size. Details of PharmGKB Level of Evidence can be found on PharmGKB.
FDA Label Testing
The FDA maintains a list of drug labels with pharmacogenomic information in their labeling: Table of Pharmacogenomic Biomarkers in Drug Labeling.PharmGKB annotates these drug labels with information about the strength of the language on the label and whether genetic testing is required, recommended or not discussed. Details of the FDA label testing descriptions can be found on PharmGKB.